Accurate monitoring of chimerism after hematopoietic stem cell transplantation using Crystal Digital PCR
Developed by:
MSc. Elise Gourri, Dr. med. Beat M. Frey, Dr. rer. nat. Stefan Meyer Blood Transfusion Service Zurich (ZHBSD)

Monitoring of chimerism in patients after stem-cell transplantation
Bone marrow and peripheral blood stem cell transplantation are well-established treatment procedures for many malignant and non-malignant disorders1,2. After transplantation of hematopoietic stem cells, the recipient generates new blood cells that genetically are of donor DNA origin. It is a part of the surveillance of impending clinical relapse that the newly developed hematopoietic system is of donor origin. The investigation of the genotypic origin in post-transplant patients is called chimerism analysis2. Full donor chimerism implies that 100% of blood cells are of donor origin, while mixed or partial chimerism means that recipient cells are also present. Thus, for monitoring purposes the respective proportion of donor and recipient DNA is measured in the blood or bone marrow of the recipient following the transplantation.
The previously established method for chimerism monitoring in the lab was based on detection of bi-allelic Single Nucleotide Variations (SNVs) by TaqMan® assays used on a chip-based digital PCR 3 (Thermofisher). It required substantial hands-on time and was not compatible with increasing sample throughput. To circumvent these limitations, the lab is now using Crystal Digital PCR™ with the Opal chip on the naica™ system (Stilla Technologies), thus attaining higher sample throughput with minimal hands-on time. Crystal Digital PCR™ combines automated sample partitioning and thermocycling on the Geode. The fluorescence signal of each droplet is then measured in the Prism3. An Opal chip allows SNV detection in up to 20,000 droplets in each of the 16 reaction units (wells), and up to 3 chips (48 reactions) can be processed in a single run.
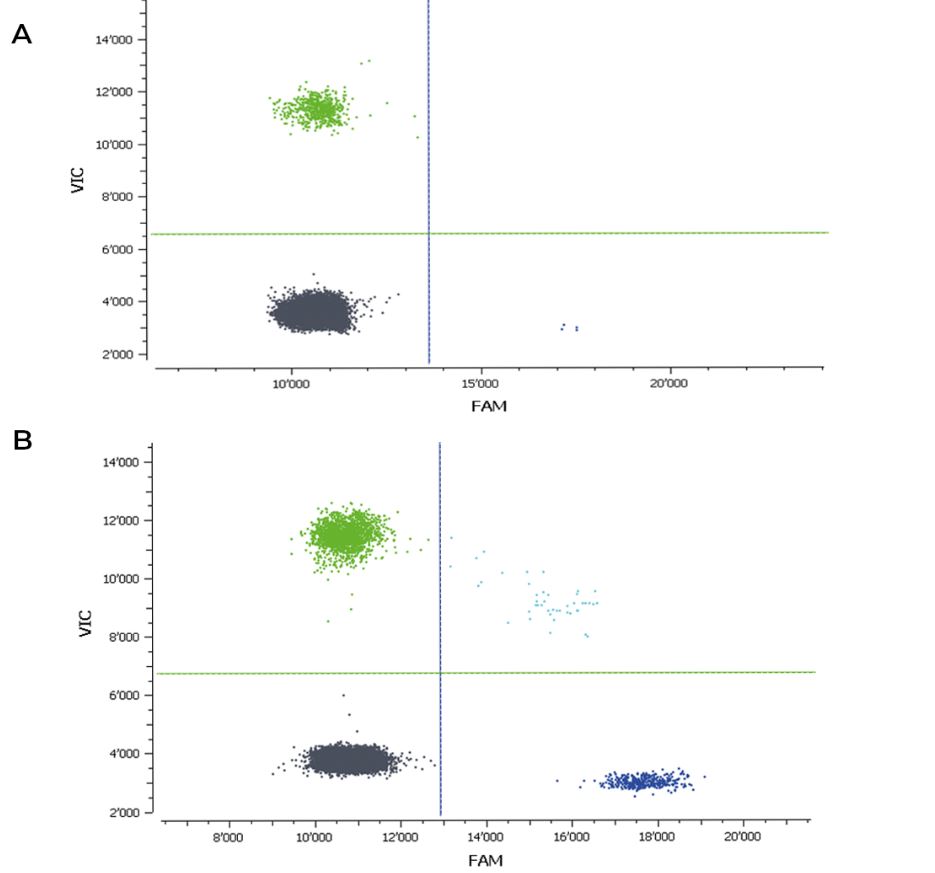
Figure 1: 2D Crystal Digital PCR™ analysis of an artificial DNA mix with SNV rs1058396 (TaqMan® assay, Thermofisher). A: Analysis of 5ng of an artificial DNA mix: DNA homozygous for allele G (FAM-labelled) mixed with DNA homozygous for the allele A (VIC-labelled). The naica™ system allowed for accurate quantification of 0.5% allele G in background of allele A. B: Analysis of the conventionally used 20ng DNA input with Crystal Digital PCR™ using an artificial DNA mix with 16% allele G in 84% allele A.
Two-step strategy for chimerism monitoring
Pre-transplant: marker selection
To enable proper post-transplant monitoring, two specific SNV assays need to be selected on pre-transplantation samples in a backup model. The lab has established a portfolio of 24 specific SNV assays to screen for unique markers that distinguish donor and recipient DNA in a single sample analysis4. Out of the 24 potential SNV assays that can be used, the two selected SNVs must show different genotypes for donor and recipient.
Post-transplant: chimerism monitoring
Clinically, a minimum of 0.5% minor allele sensitivity is desired for chimerism monitoring. Using only 5ng of input DNA, the desired 0.5% sensitivity was achieved with Crystal Digital PCR™ (Figure 1A). A reproducible quantification of a minor allele frequency even below 0.25% could be accomplished with the routinely used 20ng input DNA. An example of a standard test result is given in Figure 1B.
Testing the optimal sample volume
The Crystal Digital PCR™ analysis software (Crystal Miner) allows for the calculation of the target (SNV) concentration in copies/μl (cp/µl) using the number of positive droplets under consideration of the total number of droplets in the well applying Poisson law statistics. To estimate the sensitivity of this assay, two SNV targets were quantified with increased amounts of DNA input. As expected, an increasing number of measured cp/µl with increasing amounts of input DNA was observed. With the routine experimental setup of 20ng input DNA, approx. 650 cp/µl can be expected and used as an internal quality control (Figure 2). In addition, the reliability of the naica™ system was confirmed on previously monitored patient samples and several external proficiency test samples (EQA) (Table 1).
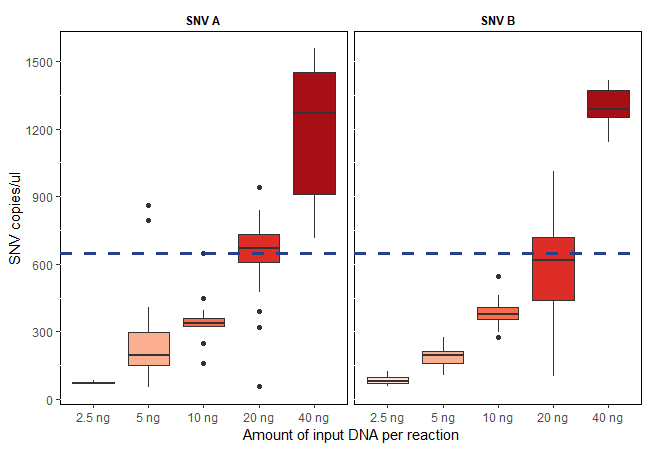
Figure 2: Total number of two different SNV targets, measured in copies/µl (cp/µl) per reaction at different DNA input amounts. TaqMan® assays specific for two different SNVs were used in 16 replicates each. As expected, an increasing number of detected cp/µl by increasing the amount of input DNA was observed. Approximately 650 cp/µl are to be expected with the routine experimental setup of 20ng input DNA per reaction.
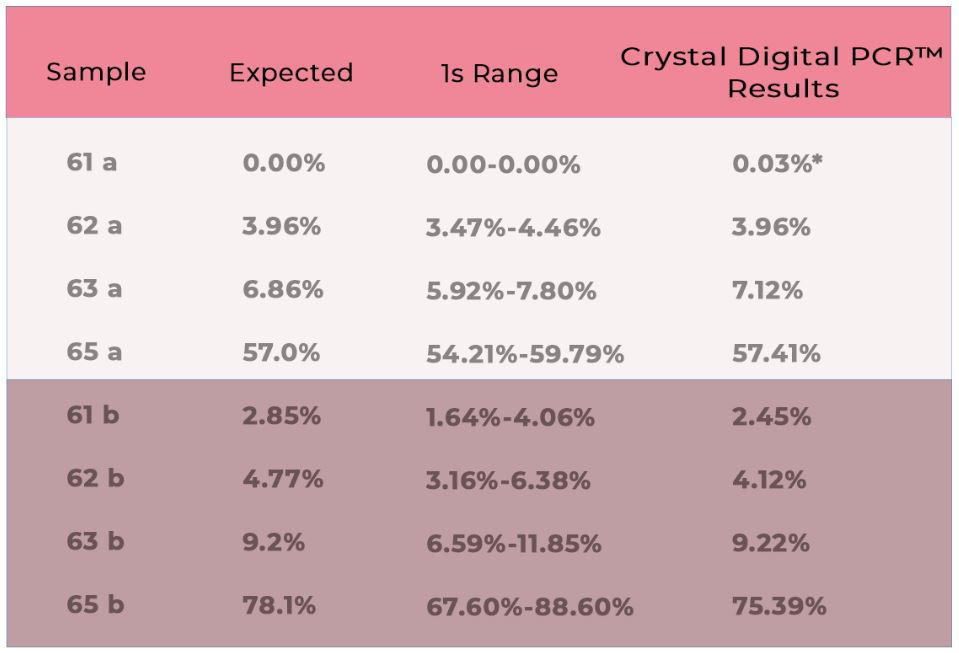
Table 1: Results of two independent chimerism EQAs from Instand e.V. samples (artificial blood mixes) from 2017 (61a-65a) and 2018 (61b-65b) were analysed in duplicate with two TaqMan® assays targeting SNVs. Results are given in percent of ʺreceiverʺ. Results within the 1s-range are awarded with the maximum grade. *0.03% corresponds to a negative result.
Application Note Highlights
- Crystal Digital PCR™ is fully compatible with the previously established SNV-based chimerism monitoring method at Blood Transfusion Service Zurich (Swiss Red Cross).
- Using Crystal Digital PCR™, the test of a standard input amount of 20ng DNA allowed a detection sensitivity down to 0.25% allele frequency, well below the minimum clinical limit of 0.5%.
- Using the Opal chips on the naica™ system requires minimal hands-on time and only a low amount of input DNA per reaction, allowing higher sample throughput and the potential for lineage specific chimerism monitoring.
- The naica™ system is a fast, convenient and highly accurate method to monitor post-transplant chimerism.
ABOUT
The Blood Transfusion Service Zurich, a member of the Swiss Red Cross, is one of the leading centers for Transfusion Medicine in Switzerland. Besides handling and testing of classical blood related products, the institution also performs molecular diagnostics of blood groups and hematological issues. Since 2015, the Department of Molecular Diagnostics has used dPCR to develop novel methods for genetic diagnostics in transfusion and transplantation medicine.
REFERENCES
1. Appelbaum FR. The current status of hematopoietic cell transplantation. Annu Rev Med 2003; 54: 491–512.
2. Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T. How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant. 2005;35:107-119.
3. Gourri et al. DGHO Annual conference 2016.
4. Boettcher et al. Clonal hematopoiesis in donors and long-term survivors of related allogeneic hematopoietic stem cell transplantation. Blood. 2020 Apr 30;135(18):1548-1559. Supplementary https://doi.org/10.1182/blood.2019003079
To learn more about digital PCR, please visit Stilla Technologies’ Learning Center.