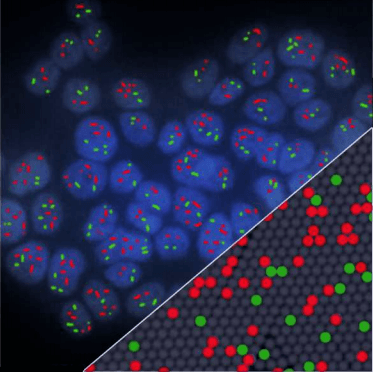
Guidelines for 3-color multiplex assay design for optimized performance with Crystal Digital PCR™
By measuring several targets in a single reaction, multiplex assays enable users to conserve precious sample volume and to save time, reagents and costs. In addition, as all targets are amplified and measured in the same reaction, multiplexing improves quantification precision by reducing pipetting errors that contribute to sample and reagent variations when performing separate single-plex reactions. Multiplexing in Crystal Digital PCR™ is as sensitive and accurate as single-plexing. Careful assay design and assay optimization steps help realize the more complex reaction composition, containing a high number of primers and probes for the amplification of several DNA targets in a single PCR reaction. The Crystal Miner software is an open data analysis software that provides intuitive tools to help optimize and troubleshoot multiplex assays. This Technical Note provides straightforward guidelines to facilitate multiplex assay design using the naica® system.
Guidelines for experimentally evaluating primer and probe performance:
Stilla® recommends using the naica® multiplex PCR MIX, which is specially formulated for optimal multiplexed Crystal Digital PCR™ performance.
- It is imperative to start with a Crystal Digital PCR™ run of the individual reaction composition in the single plex format first. Each primer/probe/target requires a performance verification in the less complex reaction mix before proceeding to multiplexing. For example, for a 3-plex assay, three individual single-plex reactions should first be performed on control nucleic acid target templates before combining the reagents in a multiplex reaction. When performing a single-plex reaction, a single positive population is expected.
- For optimized multiplex assay performance, it is important to consider the final sample matrix and composition (e.g., short fragmented DNA for assay design targeting circulating cell-free DNA, more intact DNA fragments for assay design targeting genomic DNA). The DNA template used should be devoid of contaminants and potential inhibitors. Synthetic oligos can be used as templates for assay optimization if final sample material is rare or not readily available.
- A range of elongation temperatures should be evaluated for each single-plex reaction to determine the optimal reaction temperature at which there is good separability between positive and negative populations, without non-specific amplification (Figure 1). The Stilla® separability score provided by Crystal Miner software (Figure 2) should be used as a metric to determine the optimal elongation temperature common to all probes. If the single-plex reaction is not well optimized, a second distinct amplified population may be apparent due to, for example, undesired probe interactions. In addition, non-specific amplification can result from one of several unoptimized parameters, including primer/probe dimers or primer/probe non-specificity. In this case, various methods can be employed to limit the amplification of non-specific sequences, such as increasing the annealing temperature, performing a touchdown PCR or redesigning primer sequences. It is important to evaluate the primer and probe interactions using adapted in silico tools before testing the reagents in vitro. Read More
Technical Note Highlights
- The naica® system offers flexible 3-color multiplexing capabilities.
- The Crystal Miner software facilitates multiplex assay optimization and troubleshooting.
- This technical note provides cost- and time-saving tips to design and validate Crystal Digital PCR™ multiplex assays on the naica® system.
- Designing multiplex assays is not complex if the guidelines provided in this Technical Note are followed.